USFDA RECALLS TABLETS, LIQUIDS AND INJECTABLE MEDICATIONS FROM SANDOZ, APOTEX AND GLAXOSMITHKLINE.
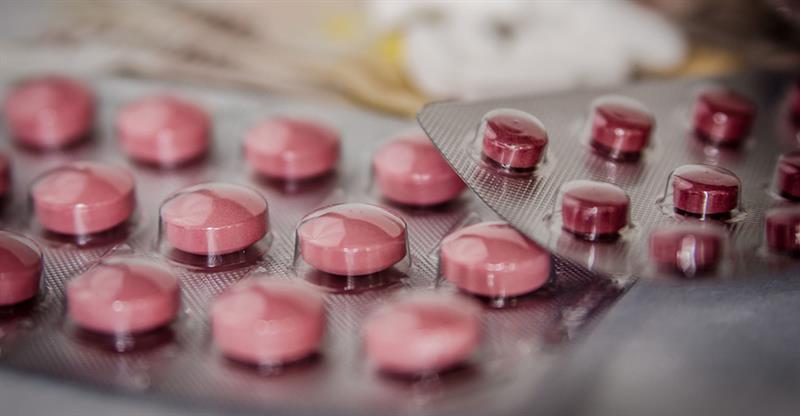 The Ministry of Health and Wellness has been informed of the recent alerts by the United States Food and Drug Administration (USFDA) and the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for the voluntary recall of Ranitidine capsules/tablets by Sandoz and Apotex and Ranitidine (Zantac) tablets, liquid and injectable preparations from GlaxoSmithKline.
The Ministry of Health and Wellness has been informed of the recent alerts by the United States Food and Drug Administration (USFDA) and the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for the voluntary recall of Ranitidine capsules/tablets by Sandoz and Apotex and Ranitidine (Zantac) tablets, liquid and injectable preparations from GlaxoSmithKline.
Our investigations have revealed that these medications from the affected manufacturers are not available in the public sector pharmacies. They are not present at Victoria Hospital, St. Jude Hospital, District hospitals and the wellness centres. However, in the private sector pharmacies specific lots from the affected batches of Zantac tablets from GlaxoSmithKline were identified and have been removed from the shelves.
The Ministry of Health and Wellness advises that persons who are unsure whether they have the product should consult their health care provider.
For any further inquiries and information, please contact the Drug Inspector, Ms. Astrid Mondesir, Ministry of Health and Wellness, Sir Stanislaus James Building, Waterfront, Castries at 468-5311.